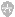EnergyDiagrams
2011-09-20 16:57:00 来源:liuxuepaper.Com 双击单词自动翻译
This energy diagram is a graph of the pro美国GREss of a chemical reaction, versus the total energy of the system. The reactant in this case is BrNO, and the products are NO and Br2. As you can see, after the reaction occurs, the energy of the system is lower than it was before the reaction. This energy diagram shows an exothermic reaction, one in which energy is given off. In the energy diagram for an endothermic reaction, the energy of the products would be higher than that of the reactants. In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. This state is also known as an activated complex. The figure below shows the energy diagram for a reaction in the presence of a catalyst and in the absence of a catalyst. As you can see, the catalyst has decreased the activation energy of the reaction, which means that more molecules are able to surmount it and react.
(责任编辑:申月月)
相关热词搜索:Energy Diagrams reaction activ
上一篇:美国GRE外语考试经典试题(5)
下一篇:SAT语法的特点和解题技巧